External Quality Assessment (EQA) is an essential aspect of any laboratory operation.
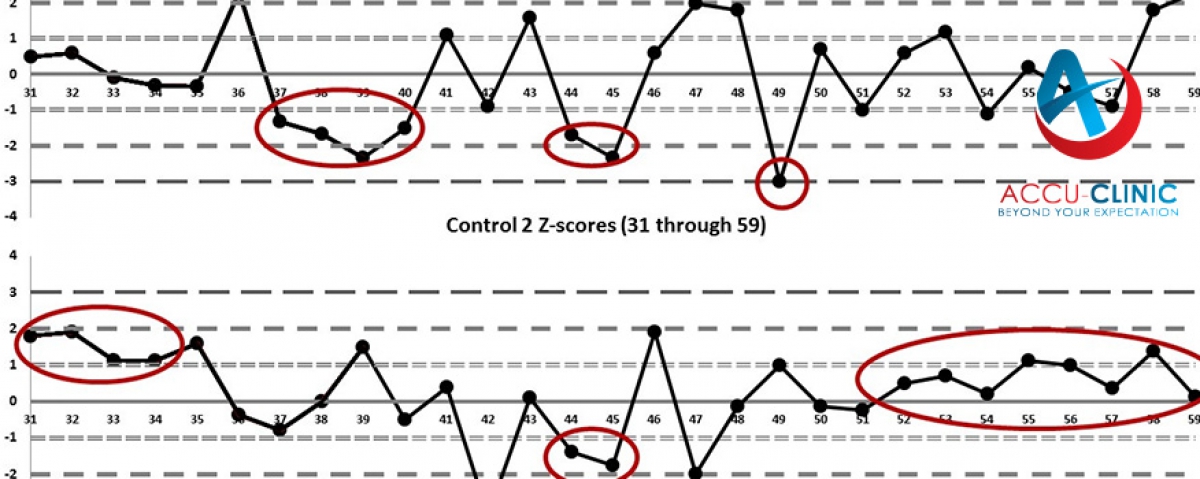
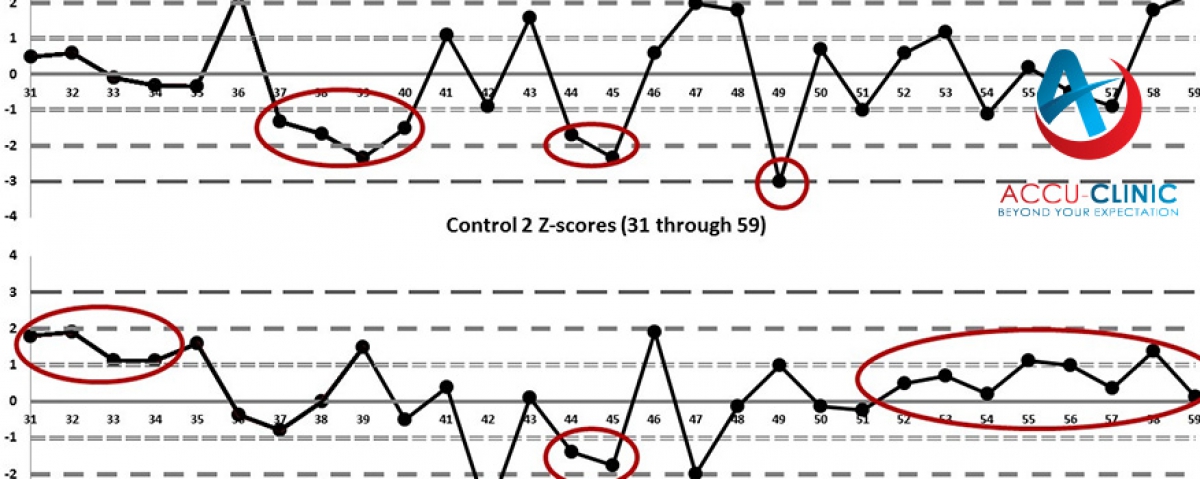
EQA provides a means of assessing the analytical performance of a laboratory compared to other laboratories utilizing the same methods and instruments.
Overall objective of EQA:
To develop inter-laboratory comparability which allows standardisation of diagnostic testing. EQA measures a laboratory's accuracy using 'blind' samples that are analysed as if they were patient samples. Results are returned to the scheme organiser for statistical analysis. Laboratories receive a report comparing their individual performance against other participants in the programme. EQA has a number of functions:
- Maintaining and improving the analytical quality of laboratory tests
- Improving inter-laboratory agreement and raising standards
- Detecting equipment failures, identifying reagent problems, reviewing staff training
- Initiating and evaluating corrective actions
- Comparing different analytical methods
Participation in an EQA scheme will help produce reliable and accurate reporting of patient results. Quality results will reduce time and labour costs, and most importantly provide accurate patient diagnosis and treatment.
A Good EQA scheme should have:
- Sufficient number of participants
- Effective consolidation of programmes
- International recognition through accreditation
- Quality material
- Regular reports with rapid turnaround times
- Independent advisory panel
- Flexible programme choices